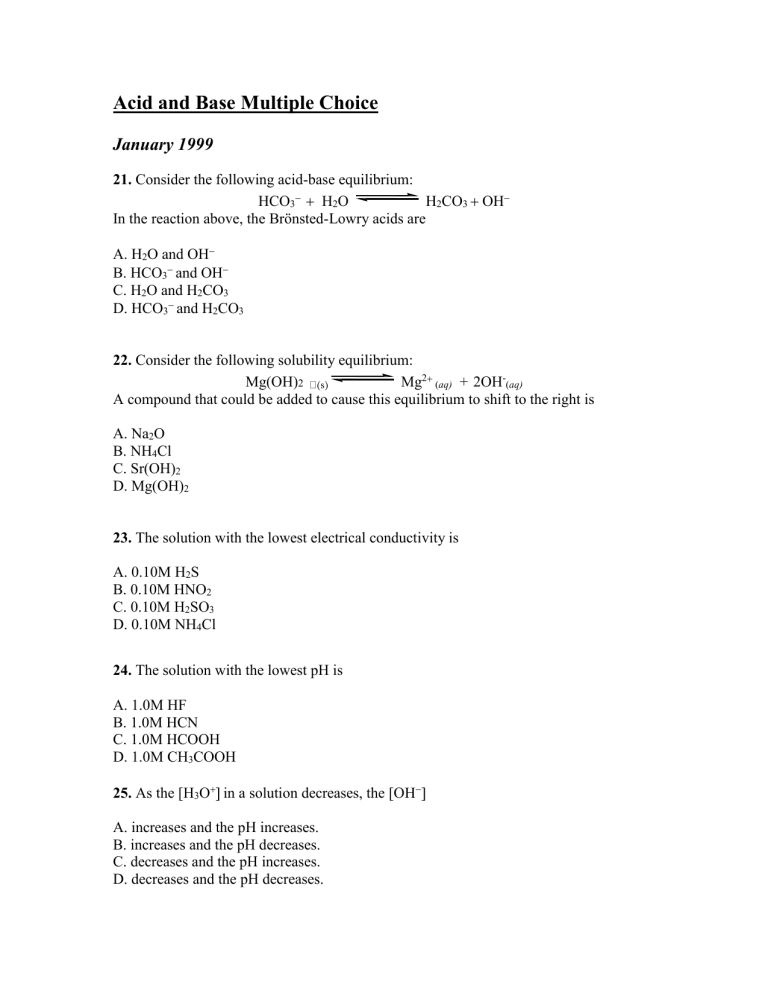
Which Equilibrium System Has Hco3- Acting As A Brønsted-lowry Base?
Which equilibrium system has hco3- acting as a brønsted-lowry base?. Complete Solutions Manual GENERAL CHEMISTRY NINTH EDITION EbbingGammon. 30 x 103 H2C4H4O6 equilibrium system where the conjugate acid and base pairs both exist ex. A Brief Review 698 Brønsted Lowry Theory of Acids and Bases 698 Self-Ionization of Water and the pH Scale 703 Strong Acids and Strong Bases 706 Weak Acids and Weak Bases 708 Polyprotic Acids 717 Ions as Acids and Bases 723 Molecular Structure and Acid Base Behavior 727 Lewis Acids and Bases 732 Summary 736 Integrative Example.
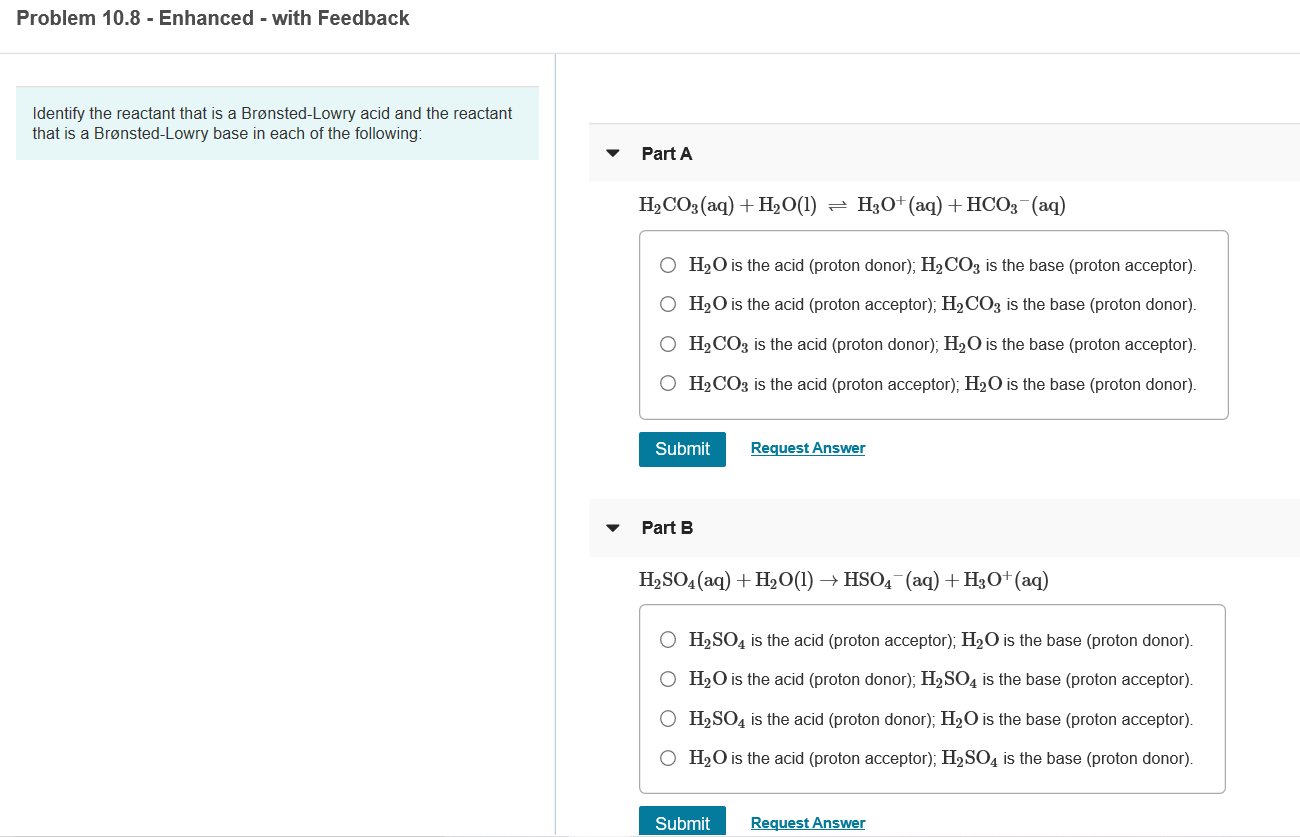
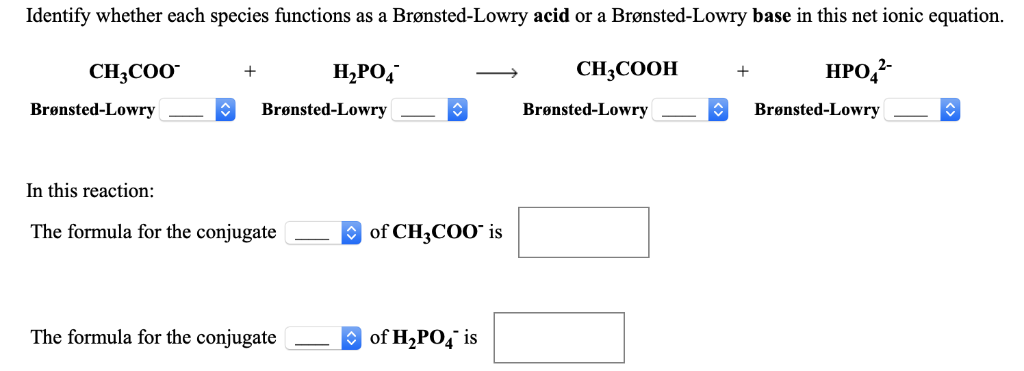
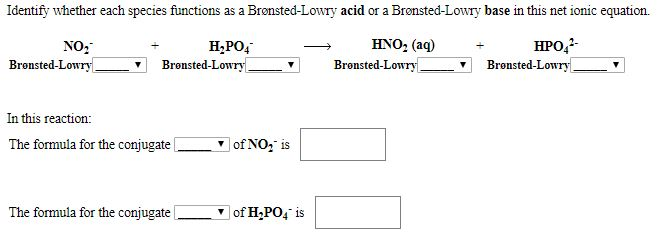
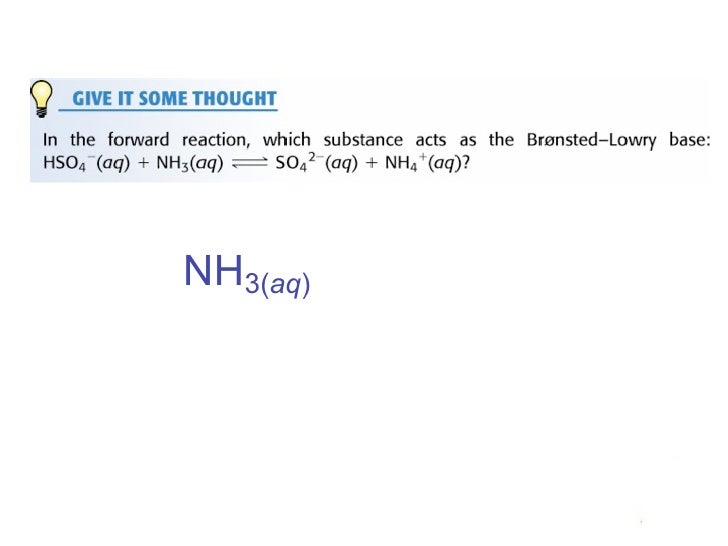
Com Part C Use the table in the introduction to compare the strength of the acid and conjugate. Cambridge International AS and A Level Chemistry Coursebook 2nd Edition. In the following net ionic equation identify each species as either a Brønsted-Lowry acid or a Brønsted-Lowry base.
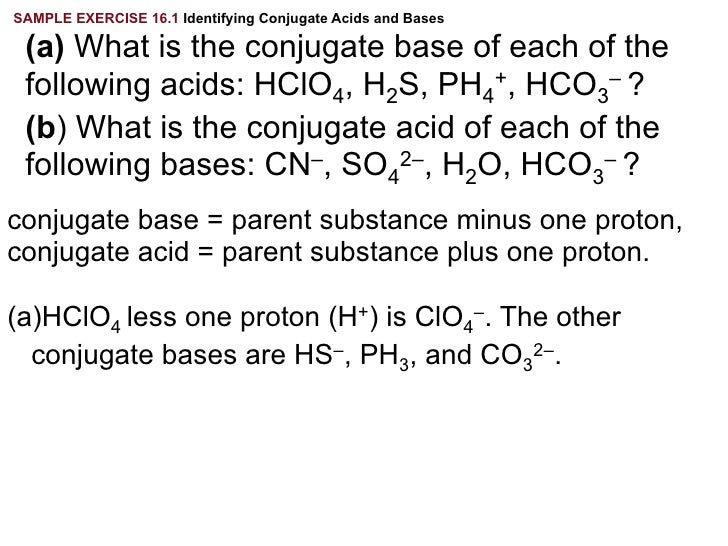
Write the formula for the conjugate acid for each of the following Relationship between Ka. Complete Solutions Manual General Chemistry Ninth Edition. Its CAS number is 7782-99-2.
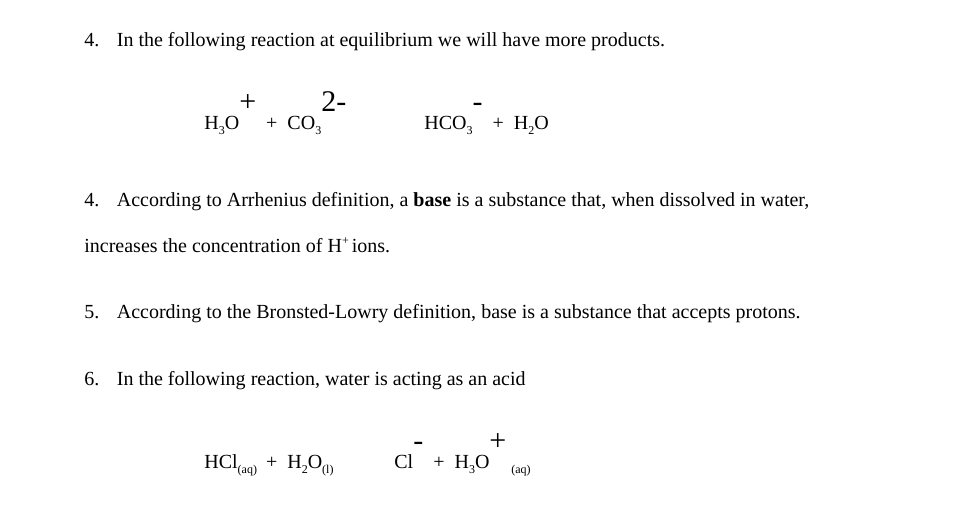
A weak acid is an acid that partially dissociates into its ions in an aqueous solution or water. HSO4- is a base since it has the ability to accept a proton but it is a conjugate base to H2SO4 since it is formed by the H2SO4 after donating a proton. HCO3- H3PO4 aq H2CO3 aq H2PO4-The formula for the conjugate of HCO3- is The formula for the conjugate of H3PO4 is HCO3- is a base H3PO4.
Enter the email address you signed up with and well email you a reset link. Cambridge International AS and A Level Chemistry Coursebook 2nd Edition. The formula for the conjugate base of h2so3 is.
HSO4- is a base since it has the ability to accept a proton but it is a conjugate base to H2SO4 since it is formed by the H2SO4 after donating a proton.
A Brief Review 698 Brønsted Lowry Theory of Acids and Bases 698 Self-Ionization of Water and the pH Scale 703 Strong Acids and Strong Bases 706 Weak Acids and Weak Bases 708 Polyprotic Acids 717 Ions as Acids and Bases 723 Molecular Structure and Acid Base Behavior 727 Lewis Acids and Bases 732 Summary 736 Integrative Example. A Brief Review 698 Brønsted Lowry Theory of Acids and Bases 698 Self-Ionization of Water and the pH Scale 703 Strong Acids and Strong Bases 706 Weak Acids and Weak Bases 708 Polyprotic Acids 717 Ions as Acids and Bases 723 Molecular Structure and Acid Base Behavior 727 Lewis Acids and Bases 732 Summary 736 Integrative Example. 30 x 103 H2C4H4O6 equilibrium system where the conjugate acid and base pairs both exist ex. Complete Solutions Manual GENERAL CHEMISTRY NINTH EDITION EbbingGammon. The formula for the conjugate base of h2so3 is. Write the formula for the conjugate acid for each of the following Relationship between Ka. In the following net ionic equation identify each species as either a Brønsted-Lowry acid or a Brønsted-Lowry base. HCO3- H3PO4 aq H2CO3 aq H2PO4-The formula for the conjugate of HCO3- is The formula for the conjugate of H3PO4 is HCO3- is a base H3PO4. Enter the email address you signed up with and well email you a reset link.
The formula for the conjugate base of h2so3 is. Write the formula for the conjugate acid for each of the following Relationship between Ka. A weak acid is an acid that partially dissociates into its ions in an aqueous solution or water. Complete Solutions Manual GENERAL CHEMISTRY NINTH EDITION EbbingGammon. In the following net ionic equation identify each species as either a Brønsted-Lowry acid or a Brønsted-Lowry base. Com Part C Use the table in the introduction to compare the strength of the acid and conjugate. Its CAS number is 7782-99-2.
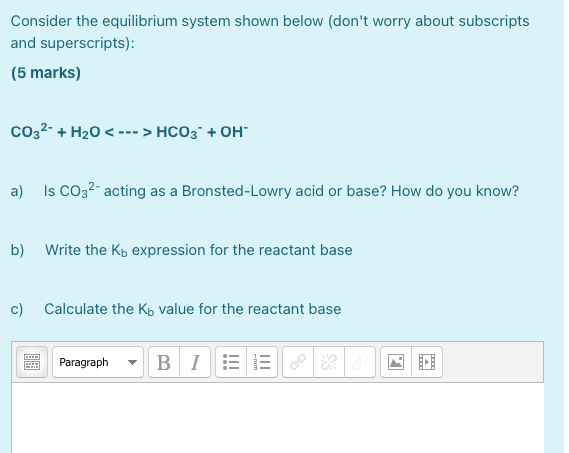
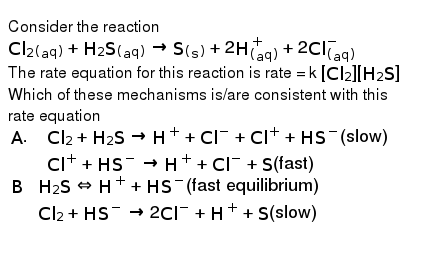
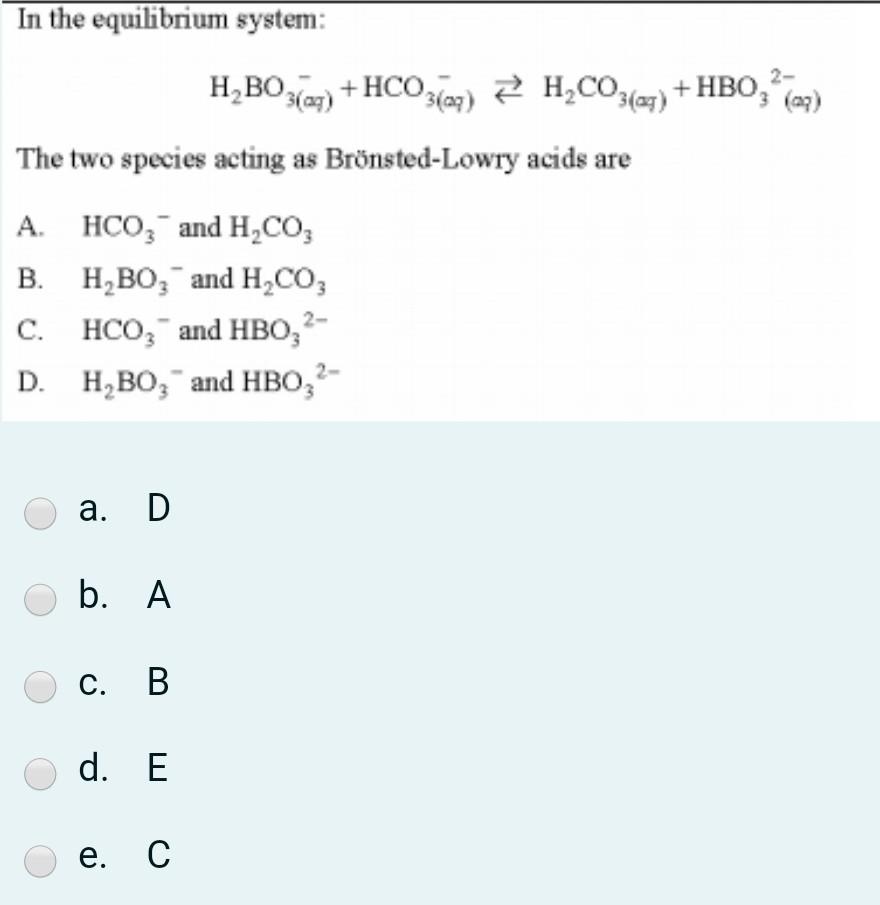


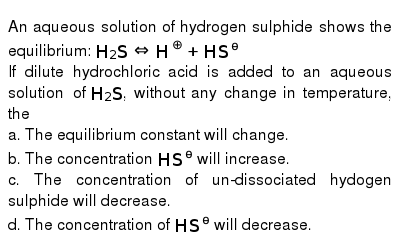
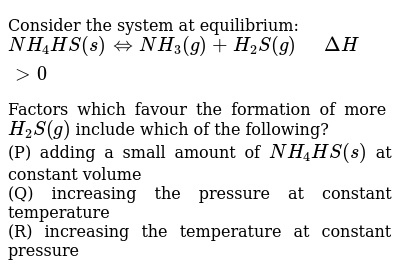



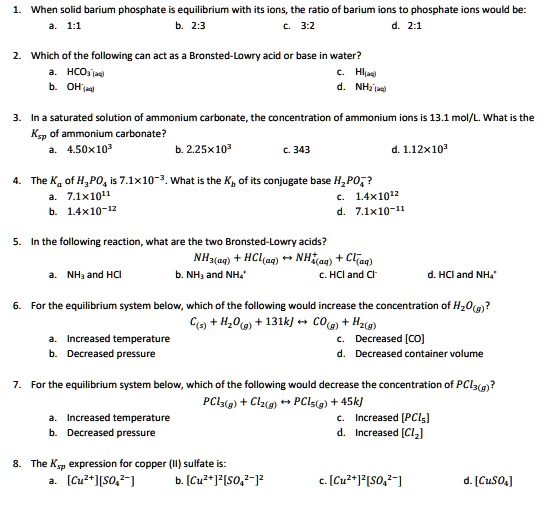
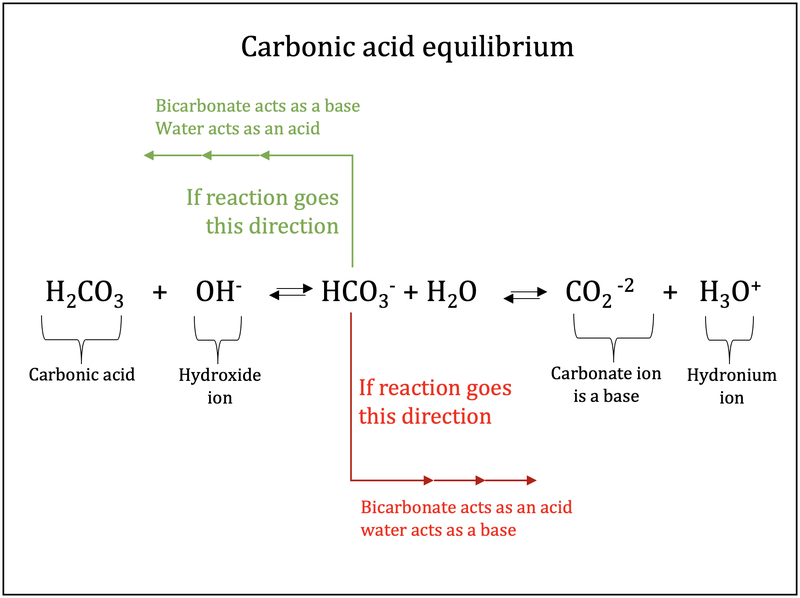
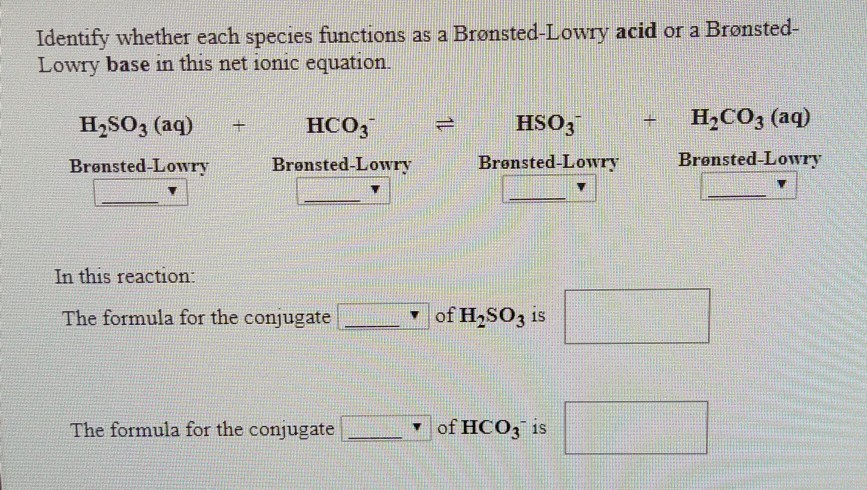
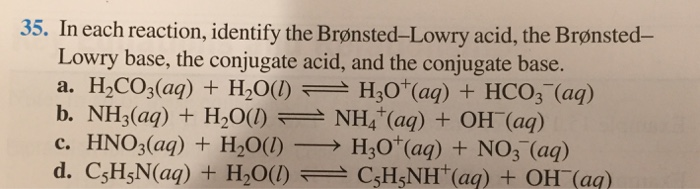
















Post a Comment for "Which Equilibrium System Has Hco3- Acting As A Brønsted-lowry Base?"